Products & Services
The key deliverables of KD Metrix are application of Design of Experiments (DoE), multivariate data analysis and multivariate strategy related to:
• Development and optimization of robust synthesis and manufacturing processes, process upscale and process transfer
• Development of drug composition (formulation)
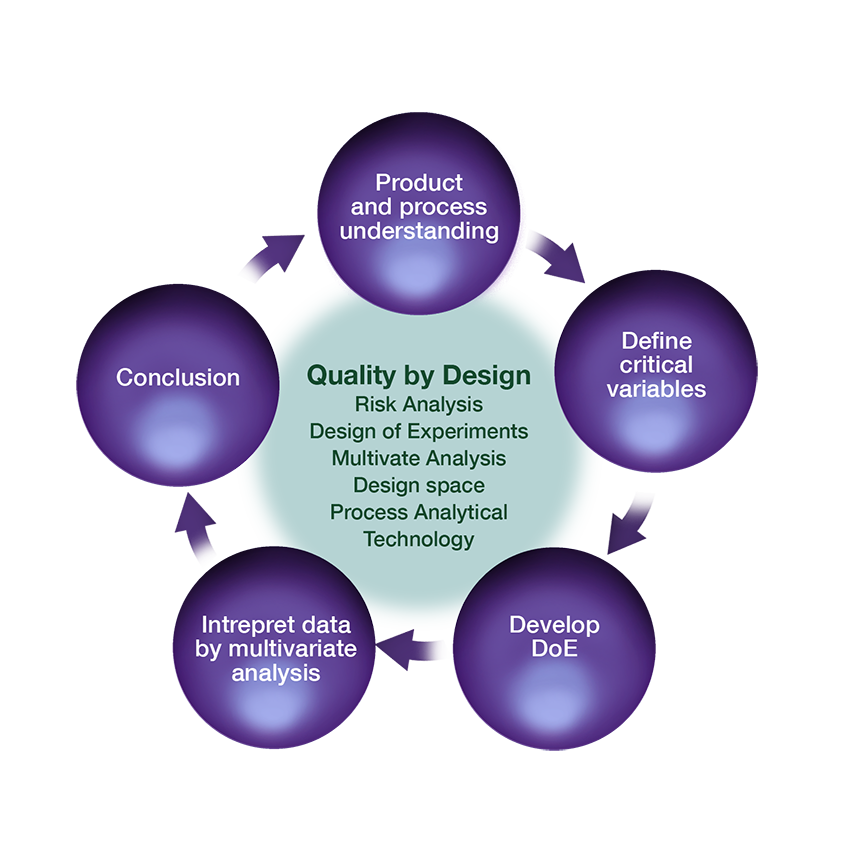
• Quality by Design (QbD) and risk analysis
• Simplified product and process control by various process analytical technology (PAT)
• Biological and clinical data, efficacy and pharmacokinetic modelling
• Drug stability studies; predict and confirm shelf life
• Analytical method development and validation of LC, TLC, GC, NIR, IR, MS, NMR, UVVIS (accuracy, precision, linearity, LOD, LOQ), including sensitive LC-DAD peak purity analysis, analysis of data from various particle monitoring instrements
• Purification by solid phase methodology
• Structure-activity modelling
The multivariate analysis is applied on data ranging from high resolution data, as DoE, to low resolution data, as compiled data, from processes and instruments listed above. The general goal is to apply and develop multivariate tools to draw important conclusions by a minimum number of experiments and observations to support any drug, process and method development.
KD Metrix can assist a wide multivariate drug development focus increasing the likelihood of elaborate critical variables in early development phases. In many situations, this is most efficiently supported by initiating collaboration between the client and KD Metrix in the early development phase.
The recent years the application of the QbD strategy including Risk Analysis (Q9) and pharmaceutical development and design space (Q8) has gained more focus, and experience from the application of Q8, Q9 and Q10 has been accumulated and is offered to customers.